1. Types and application
Bioplastics are used as alternatives to conventional fossil fuel based plastics and are increasingly being used in food contact materials (FCMs). For example, the Coca Cola Company has recently launched its Plant Bottle, which is partially made from biobased plastics and Danone is using polyactide (PLA) for its yoghurt cups. Two different types of bioplastics exist: biobased polymers and biodegradable plastics. Two further specific definitions for biorelated plastics are oxo-biodegradable plastics and bio-nanocomposites.
Biobased polymers are made from biobased resources though in practice biobased resource content may vary [1]. Biomass used for the production of bioplastics may either be extracted directly from plants (starch, cellulose) or produced by microorganisms in fermentative processes (e.g. polyhydroxyalkanoates (PHA)). Biomass can either be from 1st generation feedstock (e.g. corn, sugar cane) or from non-food crops (2nd generation feedstock, e.g. lignocellulosic material). Biobased polymers can also be produced by further chemical modifications and are not necessarily biodegradable.

Figure 1: Bioplastics – Material source and biodegradability
Biodegradable plastics may be made from both natural and fossil resources and are biodegraded by microorganisms in their natural environment. The products of this process are energy, biomass, water and carbon dioxide or methane, depending of the presence or absence of oxygen. If biodegradable plastics are degraded in accordance with standards for compostability, e.g. the European standard EN 13432, they may be labeled compostable.
Oxo-biodegradable plastics are mainly composed of polyolefins such as polyethylene (PE) and polypropylene (PP), which contain further chemical additives intended to accelerate degradation. Oxo-biodegradable plastics do not degrade according to the previously mentioned standards.
Bio-nanocomposites are biopolymers which have been stabilized using nanoparticles [2]. The nanoparticles enhance technical properties, such as barrier, thermal, chemical or mechanical stability and include nanoclays and nanosilver [3]. The following bioplastics, which are to a varying degree biobased and biodegradable, are relevant for FCMs (Figure 1) [4]:
| Starch-based polymers | - Biodegradable polysaccharide - Alternative for polystyrene (PS) - Used in food packaging, disposable tableware and cutlery, coffee machine capsules, bottles |
| Cellulose-based polymers | - Biodegradable polysaccharide - Low water vapor barrier, poor mechanical properties, bad processability, brittleness (pure cellulosic polymer) - Regulated under 2007/42/EC - Coated, compostable cellulose films - Used in the packaging of bread, fruits, meat, dried products, etc. |
| Polylactide (PLA) | - Biodegradable, thermoplastic polyester - Possible alternative of low- and high-density polyethylene (LDPE and HDPE), polystyrene (PS), and poly terephthalate (PET) - Transparent, rigid containers, bags, jars, films |
| Polyhydroxyalkanoates (PHA) | - Biodegradable polyester - Family of many, chemically different polymers - Brittleness, stiffness, thermal instability |
| Biobased polypropylene (PP) and polyethylene (PE) | - Non-biodegradable vinyl polymer - Mainly based on sugar cane - Identical physicochemical properties |
| Partially biobased polyethylene terephthalate (PET) | - Alternative to conventional PET - Up to 30% biobased raw materials - Used in bottles |
| Biobased polyethylene furanoate (PEF) | - Non-biodegradable polyester based on a heteroaromatic 5-ring structure - Better barrier function than PET - Up to 100% biobased raw materials - May be used in the future in bottles, fibers, films |
| Aliphatic (co)polyesters | - Biodegradable polymers including e.g. polybutylene succinate (PBS), polyethylene succinate (PES), and polyethylene adipate (PEA) - Used in disposable cutlery |
| Aliphatic-aromatic (co)polyesters | - Biodegradable polymers including e.g. polybutylene adipate terephthalate (PBAT), polybutylene succinate terephthalate (PBST). - Used as fast food disposable packaging, PBAT for plastic films |
| Polycaprolactone (PCL) | - Biodegradable polyester - Low melting temperature, easily biodegradable - Used in medical applications, as PCL blends in FCMs |
| Polyvinyl alcohol (PVOH) | - Biodegradable vinyl polymer - Used for coatings, adhesives, and as additive in paper and board production |
| Polyamides (PA) | - Non-biodegradable polymer - Used in high-performance polymers, not commonly in FCMs |
| Others | - Animal (chitosan) and protein (soy protein isolate, gluten and zein) based bioplastics |
Usually pure biodegradable plastics do not perform as well as conventional plastics. Material properties are enhanced by the addition of chemicals including antioxidants, light and UV stabilizers, releasing agents, cross-linking agents and many others.
2. Toxicity
The monomers of cellulose- and starch based polymers as well as those of PHB and PLA are judged to be of no health concern [5, 6]. This stands in contrast to the toxicological properties of many other monomers used in plastics packaging materials (e.g. bisphenol A (BPA), bisphenol S (BPS), vinyl chloride and acrylamide). However, more additives are usually used in bioplastics than in conventional plastics. The different physicochemical properties of biobased FCMs may result in higher or lower migration rates of additives. Pure bioplastics are usually less stable and have a lower diffusion barrier than conventional plastics. Migration from PLA and starch-based polymers was reported to be low [3, 5, 7]. From oxo-biodegradable plastics, degradation aids are a further source of chemical migrants, in addition to other additives. In their 2011 publication, Ammala and colleagues provide an overview of commercial and potential degradation aids, including benzophenones and di-thiocarbamates [8]. Regarding bio-nanocomposites, 3 of 4 studies showed cytotoxic effects of nanoclays [9-12]. However, these studies do not allow for a conclusion on the toxic potential of bio-nanocomposites due to the great variation among nanomaterials, even those derived from the same batch, and a lack of standardized testing conditions.
3. Market, regulation and standards
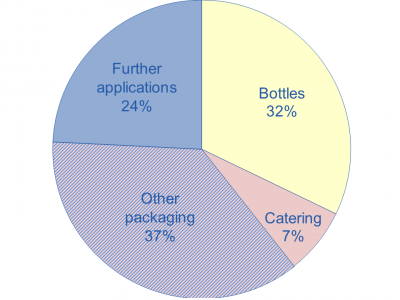
Bioplastics covers approximately 1% of the global plastics demand according to the European Bioplastics association and Plastics Europe. In 2007, starch had 50% of the market share of bioplastics, followed closely by PLAs [4]. Applications of bioplastics are distributed as shown in Figure 2 [13].
While currently only 0.01% of arable land mass is used for cultivation of raw materials used for bioplastics, the substitution of all plastics with bioplastics would require the use of 7% of the globally arable land. Plastics Europe and European Bioplastics suggest the use of sustainability certification schemes to ensure sustainable sourcing. Opponents have argued that the cultivation of crops for bioplastics requires very intensive farming including the use of fertilizers, pesticides, high water usage and possibly genetically modified plants considered inconsistent with sustainable agriculture.
Various labels are available to certify a bioplastic product. Labels certify a material’s potential of biodegradability or compostability under the conditions defined in the corresponding standards. As landfills used for garbage disposal usually provide anaerobic conditions many biological processes required for decomposition are prevented. Another concern expressed regarding biodegradable plastics is the potential accumulation of metabolites in industrial composting facilities. The testing of the final compost for heavy metals and other toxic chemicals is required, but may be difficult and expensive.
The use of Life Cycle Assessment (LCA) to evaluate the environmental impact of packaging materials, including bioplastics, has provided highly variable results, as a result of the variety of criteria and assumptions applied. Generally, it is important to consider as many environmental impact categories as possible when choosing an alternative material using LCA. Usually, bioplastics score lower in energy and carbon dioxide equivalents, but have a higher eutrophication potential. Under standard LCA methods, chemical migration and subsequent human health effects during the actual use phase are currently not considered.
In Europe, bioplastics are regulated under Commission Regulation EC 10/2011 and substances used in the manufacturing of bioplastics must be listed on Annex I of the regulation. Regenerated cellulose films are specifically regulated under Commission Directive 2007/42/EC. Substances used in nanoform require an explicit authorization according to article 9, EC 10/2011. Currently carbon black, titanium nitride and silicon dioxide are authorized as nanoparticles for use in plastic FCMs. Under US food contact law, bioplastics are regulated in the same manner as other indirect food contact materials (see also FPF article on FCM regulation). The US food law does not yet distinguish in its authorizations between the nano- or macroform of a substance. However, the use of nanoclay in food contact is considered generally recognized as safe (GRAS) in the US.
In addition to regulation, standards on compostability, biodegradability and the determination of the biobased carbon content exist. Certification organizations like DIN CERTCO, Vincotte, Bioderadable Products Institute and Japan BioPlastics Association verify claims regarding the biodegradability or compostability and allow the labeling with the respective logo, including the “seedling” logo, “OK compost” and other logos specifying the percentage of biobased content.
4. References
1. Vert, M. et al. (2012). “Terminology for biorelated polymers and applications (IUPAC Recommendations 2012).” Pure Appl Chem. 84:377-410.
2. Rhim, J.-W. et al. (2013). “Bio-nanocomposites for food packaging applications.” Prog Polym Sci. 38:1629-52.
3. Avella, M. et al. (2005). “Biodegradable starch/clay nanocomposite films for food packaging applications.” Food Chem. 93:467-74.
4. Niaounakis, M. (2013). “Biopolymers: Reuse, Recycling and Disposal.” Elsevier, Amsterdam.
5. Conn, R.E. et al. (1995). “Safety assessment of polylactide (PLA) for use as a food-contact polymer.” Food Chem Toxicol. 33:273-83.
6. Zhu, J. et al. 2014. “Structural changes and triacetin migration of starch acetate film contacting with distilled water as food simulant.” Carbohydr Polym. 104:1-7.
7. Clarke, K. et al. 2012. “Oral 28-day and developmental toxicity studies of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate.” Regul Toxicol Pharmacol. 63:196-208.
8. Ammala, A. et al. 2011. “An overview of degradable and biodegradable polyolefins.” Prog Polym Sci. 36:1015-49.
9. Houtman J, Maisanaba S, Gutiérrez-Praena D, et al. 2014. “Toxicity assessment of organomodified clays used in food contact materials on human target cell lines.” Appl Clay Sci. 90:150-8.
10. Maisanaba S, Puerto M, Pichardo S, et al. 2013. “In vitro toxicological assessment of clays for their use in food packaging applications.” Food Chem Toxicol. 57:266-75.
11. Sharma AK, Schmidt B, Frandsena H, et al. 2010. “Genotoxicity of unmodified and organo-modified montmorillonite.” Mut Res. 700:18-25.
12. Li PR, Wei JC, Chiu YF, et al. 2010. “Evaluation on cytotoxicity and genotoxicity of the exfoliated silicate nanoclay.” ACS Appl Mater Interfaces. 2:1608-13.
13. European Bioplastics. 2014.