1. Definition and types of NIAS
Non-intentionally added substances (NIAS) are chemicals that are present in a food contact material (FCM) or food contact article (FCA) but have not been added for a technical reason during the production process. Many NIAS can migrate from the FCM or FCA into food, but it is very difficult to completely understand and control such processes. The term NIAS was introduced for plastic FCMs in Europe in the legal context (Commission Regulation (EU) No 10/2011). However, NIAS are not limited to plastics but also occur in all other non-plastic FCMs.
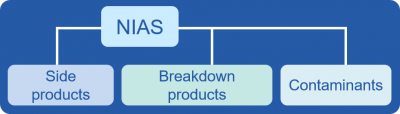
NIAS have various sources and can be grouped into side products, breakdown products, and contaminants. Side products are often formed during the production of starting substances and all further manufacturing stages. Polymerization side products derived from manufacturing of can coatings are typical examples of such reactions (FPF reported). Structure-providing constituents of FCMs (e.g., polymers, fibers) as well as additives (e.g., antioxidants, UV-stabilizers) are often degraded during manufacture and use, thus leading to various different breakdown products. Contaminants may have very different sources: Starting substances used in the production of FCMs often contain impurities or environmental contaminants which may remain in the final FCA. Processing and especially recycling can also introduce many different contaminants in FCMs and FCAs. Typical recycling-related NIAS are mineral oil hydrocarbons (MOHs), bisphenols, phthalates, and photoinitiators in recycled paper and board as well as flavor compounds, oligomers, and additives in recycled plastics (FPF reported).
2. Analysis of NIAS
Many FCMs and FCAs have a high chemical complexity making a complete characterization of all NIAS unrealistic and the identification of those NIAS that may be of concern very challenging. NIAS may be predicted based on the knowledge of chemical processes, manufacturer’s experience, and conditions of use. Such substances may then be identified and quantified rather easily by targeted chemical analyses. By using non-targeted screening methods, additional NIAS may be detected and at least some of them identified, while others remain completely unknown.
The FCM itself, a migrate or extract can be analyzed or screened for predicted and unpredicted NIAS. Thermal desorption techniques are used to directly investigate FCMs and solid food simulants. Although these methods are quick, they result in complicated fragmentation patterns that are difficult to interpret. Therefore, extracts or migrates of FCMs are typically prepared and used for further separation. Depending on the analytes, gas or liquid chromatography is applied, which is generally connected to mass spectrometry, flame ionization, ultraviolet and/or fluorescence detectors. After thorough analysis, the combination of all available information may allow to assign chemical structures to NIAS. However, many substances still remain unidentified. Quantification of NIAS is also challenging because analytical standards are usually missing. Therefore, concentrations are estimated by comparing peak areas with one or several internal standards. When using detectors optimized for “uniform” responses, prediction error ranges can differ by factors between 3 and 6.
3. Regulations
Europe
It is in accordance with the current European legislation that also non-authorized substances are present in plastic FCMs when they are non-intentionally added (Article 6(4), (EU) No 10/2011), but the FCM manufacturer is obliged to ensure NIAS safety, according to Article 3 of the Framework Regulation ((EC) No 1935/2004) and Article 19 of the Plastics Regulation ((EU) No 10/2011). Consequently, the safety of NIAS has to be assessed. At the moment, no levels of migration or exposure are set for which compliance with this requirement can be demonstrated. As specified in Regulation (EU) No 10/2011, unauthorized substances may be used in plastic FCMs behind a functional barrier, provided they do not migrate at levels above 10 µg/kg food. Substances that are known to be carcinogenic, mutagenic or toxic for reproduction (CMR) or have nanomaterial properties may not be used accordingly. Therefore, in practice a threshold of 10 µg/kg food is often used for NIAS.
United States
Any food contact substance (FCS) that is reasonably expected to migrate into food because of its intended use in an FCA must comply with the legal requirements. The definition for FCS does not cover substances that are non-intentionally added, and the term NIAS is not used in a legal context in the U.S.. However, there are provisions concerning impurities (21 CFR 174.5) and substances originating from direct contact between an FCS and the food (21 CFR 170.3(i)). The U.S. Food and Drug Administration (FDA) recommends including information on the major impurities (e.g., residual starting materials, byproducts, degradations products) when submitting a food contact notification or food additive petition for an FCS.
China
A definition for NIAS is provided in Standard GB 4806.1. Manufacturers shall perform a risk assessment to assess the safety of NIAS, but explicit approvals are not foreseen.
4. Handling the risks of NIAS
In Europe, no clear advice is given by authorities how the risk of NIAS shall be assessed. As a consequence, different industry-associated groups have published guidance documents. Accordingly, the risk assessment shall be based on information collection, chemical analysis, hazard identification and characterization, and exposure assessment. The transfer of relevant information through the supply chain (upstream and downstream) has been recognized as basic requirement facilitating the identification of major NIAS. Subsequently, the hazards of NIAS need to be addressed. A variety of tools and approaches is available for this purpose and can be used individually or in combination: (1) The classical approach evaluates toxicity data for a single, identified NIAS. (2) In silico methods rely on modeling data and can be further supported by read-across; however, they are also only applicable for identified NIAS. (3) Bioassays allow the hazard assessment of complete migrates/extracts. (4) The threshold of toxicological concern (TTC) concept assigns human exposure thresholds to NIAS and can be used for prioritization purposes. Exposure assessment is equally important and generally based on migration and consumption data. Actual migration testing, worst-case calculations, and modelling help to generate migration data, whereas information about the consumption may be obtained from standardized exposure models or specific databases.
However, hazard and exposure assessment of NIAS often relies on estimations, because relevant data are not available. Therefore, different risk assessment approaches have been proposed which are based on the available level of information. NIAS with structural information may be quantified and their hazard and exposure may be assessed by applying one or several of the above-mentioned strategies. According to classical risk assessment approaches, a NIAS is of no concern if exposure is below a hazard-based reference concentration. NIAS with unknown structure and undetected NIAS could generate a response in in vitro bioassays that allows first estimates of their safety. Under very specific conditions, the TTC concept may also help to assign exposure thresholds to unidentified NIAS.
5. Conclusions and challenges
With increasing complexity of FCMs and FCAs, NIAS will continue to be an important topic. Advances in analytical techniques and growing databases steadily facilitate the detection and identification of NIAS, but a comprehensive analysis of all NIAS is not yet in sight. International authorities recognized the importance of a risk assessment for NIAS but have not provided official guidance so far, making it difficult to enforce and comply with the legal requirements. Therefore, strategies for the risk assessment of NIAS have been developed and improved by different stakeholders in the past years. Most approaches focus on the risk assessment of single substances by in vivo, in vitro or in silico methods, but in vitro testing of the whole migrate or extract is also recommended. Additionally, robust exposure models and sensitive methods to exclude chemicals of concern are needed. Regardless of the applied concept for risk assessment, communication within the whole supply chain is essential to facilitate the prediction, identification, and quantification of NIAS.
6. Selected references
Definition and types of NIAS, including examples
- Alin J, and Hakkarainen M. 2011. Microwave heating causes rapid degradation of antioxidants in polypropylene packaging, leading to greatly increased specific migration to food simulants as shown by ESI-MS and GC-MS. J Agric Food Chem. 59:5418-27.
- Bignardi C, Cavazza A, Lagana C, et al. 2017. Release of non-intentionally added substances (NIAS) from food contact polycarbonate: Effect of ageing. Food Control. 71:329-35.
- BMELV. 2012. Ausmaß der Migration unerwünschter Stoffe aus Verpackungsmaterialien aus Altpapier in Lebensmitteln. (pdf)
- Bradley EL, and Coulier L. 2007. An investigation into the reaction and breakdown products from starting substances used to produce food contact plastics. CSL York. (pdf)
- Félix JS, Isella F, Bosetti O, et al. 2012. Analytical tools for identification of non-intentionally added substances (NIAS) coming from polyurethane adhesives in multilayer packaging materials and their migration into food simulants. Anal Bioanal Chem. 403:2869-82.
- Geueke B, Groh K, Muncke J 2018. Food packaging in the circular economy: Overview of chemical safety aspects for commonly used materials. J Clean Prod, 193:491-505.
- Geueke B. 2016. FPF Dossier: Can coatings. Food Packaging Forum. DOI: 10.5281/zenodo.200633 (pdf)
- Grob K, Biedermann M, Scherbaum E, et al. 2006. Food contamination with organic materials in perspective: packaging materials as the largest and least controlled source? A view focusing on the European situation. Crit Rev Food Sci Nutr. 46:529-35.
- Nerin C, Alfaro P, Aznar M, et al. 2013. The challenge of identifying non-intentionally added substances from food packaging materials: A review. Anal Chim Acta. 775:14-24.
- Schaefer A, Ohm VA, and Simat TJ. 2004. Migration from can coatings: Part 2. Identification and quantification of migrating cyclic oligoesters below 1000 Da. Food Addit Contam A. 21:377-89.
Identification and analysis of NIAS
- Ackerman LK, Noonan GO, and Begley TH. 2009. Assessing direct analysis in real-time-mass spectrometry (DART-MS) for the rapid identification of additives in food packaging. Food Addit Contam A. 26:1611-8.
- Bentayeb K, Batlle R, Romero J, et al. 2007. UPLC-MS as a powerful technique for screening the nonvolatile contaminants in recycled PET. Anal Bioanal Chem. 388:1031-8.
- Brenz F, Linke S, and Simat T. 2017. Qualitative and quantitative analysis of monomers in polyesters for food contact materials. Food Addit Contam A. 34:307-19.
- Nerin C, Alfaro P, Aznar M, et al. 2013. The challenge of identifying non-intentionally added substances from food packaging materials: A review. Anal Chim Acta. 775:14-24.
- Peters RJB, Groeneveld I, Lopez Sanches P, et al. 2019. Review of analytical approaches for the identificaton of non-intentionally added substances in paper and board food contact materials. Trends Food Sci Tech. DOI: 10.1016/j.tifs.2018.12.010
- Pieke EN, Granby K, Trier X, et al. 2017. A framework to estimate concentrations of potentially unknown substances by semi-quantification in liquid chromatography electrospray ionization mass spectrometry. Anal Chim Acta. 975:30-41.
- Pieke EN, Smedsgaard J, and Granby K. 2017. Exploring the chemistry of complex samples by tentative identification and semi-quantification: a food contact material case. J Mass Spectrom. 53:323-35.
- Qian S, Ji H, Wu XX, et al. 2018. Detection and quantification analysis of chemical migrants in plastic food contact products. PLoS ONE 13(12): e0208467.
- Wang Z, Wiesinger H, Groh K. 2021. Time to Reveal Chemical Identities of Polymers and UVCBs. Environmental Science & Technology. 55(21):14473–14476.
Handling the risks of NIAS
- Bengtström L, Rosenmai AK, Trier X, et al. 2016. Non-targeted screening for contaminants in paper and board food-contact materials using effect-directed analysis and accurate mass spectrometry. Food Additives & Contaminants: Part A. 33:1080-93.
- 2017. EuPIA guidance on migration: Test methods for the evaluation of substances in printing inks and varnishes for food contact materials. (pdf)
- Groh KJ, and Muncke J. 2017. In vitro toxicity testing of food contact materials: State-of-the-art and future challenges. Compr Rev Food Sci F. 16:1123-50.
- Koster S, Bani-Estivals M-H, Bonuomo M, et al. 2016. Guidance on best practices on the risk assessment of non-intentionally added substances (NIAS) in food contact materials and articles. ILSI Europe Report Series.
- Koster S, Rennen M, Leeman W, et al. 2014. A novel safety assessment strategy for non-intentionally added substances (NIAS) in carton food contact materials. Food Addit Contam A. 31:422-43.
- 2014. Risk assessment of non-listed substances (NLS) and non-intentionally added substances (NIAS) under article 19 of Commission Regulation (EU) No 10/2011 of 14 January 2011 on plastic materials and articles intended to come into contact with food. (pdf)
