The use of per- and polyfluoroalkyl substances (PFAS) as grease and stain repellents in consumer products started in the 1950 [1]. Common properties of PFAS include thermal and chemical stability as well as high water and oil repellency [2]. These characteristics make PFAS especially suitable for the use in a variety of food contact materials (FCMs). However, PFAS are highly stable against degradation in biota and the environment [3]. The high persistence and extensive application contribute to the ubiquitous presence of PFAS.
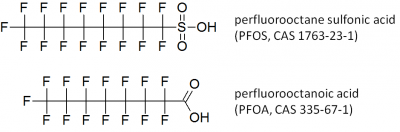
Two industrially important PFAS are perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) (Figure 1). Over decades, PFOS, PFOA and their derivatives have been widely applied, but their general persistence, low elimination rates in humans and associated adverse health effects led to different initiatives aiming at their phase-out. Nevertheless, PFAS with shorter fluorinated side chains and other types of fluorinated substances are currently used as substitutes for PFOS and PFOA [4, 5]. Short-chain PFAS have faster elimination rates in humans than their long-chain homologues, but recent research indicated that they are similarly persistent and toxic compared to their long-chain homologues [6].
1. Chemistry
The variety of organic fluorine molecules is diverse and thousands of different PFAS have been synthesized in the last decades [7, 8]. Chemical bonds between carbon and fluorine atoms are very strong leading to high thermal stability, chemical resistance and general persistence of such molecules.
PFAS
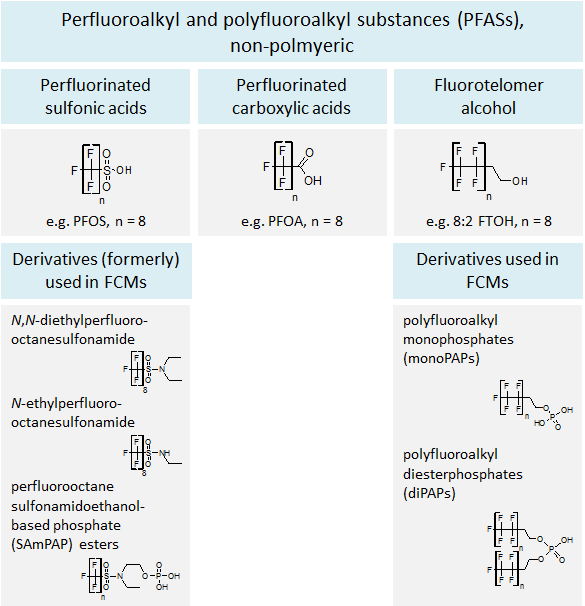
Perfluoroalkyl and polyfluoroalkyl substances (PFAS) are aliphatic substances containing, as a minimum requirement, one terminal carbon atom on which all hydrogen substituents have been replaced by fluorine atoms. Perfluorinated substances are molecules in which all hydrogen atoms bound to a carbon chain are replaced by fluorine, but functional groups in the same molecule may contain hydrogen atoms. In contrast, polyfluorinated substances are highly fluorinated, but the backbone still contains (partially) hydrogenated carbon atoms.
PFAS form a heterogeneous group of chemicals that consist of a per- or polyfluorinated carbon chain and often contain a hydrophilic functional group or moiety. Perfluorinated sulfonic acids (PFSAs), perfluorinated carboxylic acids (PFCAs), fluorotelomers, and their derivatives represent commonly used PFAS (Figure 2).
Fluorinated polymers
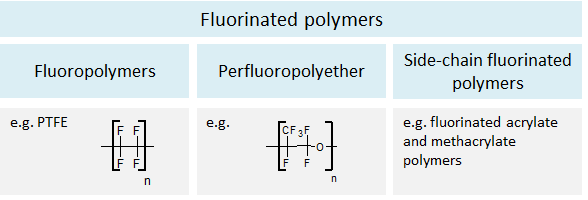
Monomers with carbon-fluorine bonds are constituents of fluorinated polymers. The best known example is polytetrafluoroethylene (PTFE, CAS 9002-84-0, trade name: Teflon®), which is composed of tetrafluoroethylene units and belongs to the subgroup of fluoropolymers (Figure 3). Perfluoropolyethers and side-chain fluorinated polymers form two further subgroups of fluorinated polymers.
More chemical structures of different types of PFAS are accessible here.
2. Applications
PFAS increase the non-stick properties of materials, as well as the repellency of oil, grease and water[4, 9]. Popcorn bags, fast food wrappers, pizza boxes, and other types of packaging that need to be oil-repellent and/or heat resistant are often coated with PFAS and represent a typical field of application. Some long-chain PFAS, e.g. PFOS, its precursors and PFOA, have been targeted by different phase-out initiatives within the last years [12-14]. As a consequence, the use of some PFOS derivatives (e.g. perfluorooctane sulfonamidoethanol-based phosphate (SAmPAP) esters) in paper and board FCMs has mainly stopped by 2002 [15]. In the recent years, fluorotelomer-based derivatives (e.g. polyfluoroalkyl phosphate esters (PAPs)), short- and medium-chain PFAS and fluorinated polymers have gradually started to replace many PFOS-based substances in FCMs [4, 9, 10].
Fluorinated polymers are commonly used as coating for pans and other kitchen utensils. In particular, PTFE-coated kitchenware can be found in many households worldwide. During the production of PTFE, PFAS are added as emulsifiers, which remain in the polymer after production, but can be removed by sintering the material before use.
3. Regulation
Restrictions
Several attempts were undertaken by authorities and industry to restrict the production and use of PFOS, PFOA and its precursors [12-14]. Between 2000 and 2002, chemical company 3M, the main producer of PFOA, PFOS and PFOS-related products, has completely phased-out these chemicals [16]. In 2006, the 2010/2015 PFOA Stewardship Program was initiated by the U.S. Environmental Protection Agency (U.S. EPA) and eight major PFAS manufacturers aiming at a strong reduction of PFOA emissions and use [12]. In 2009, PFOS and its derivatives were listed as persistent organic pollutants (POPs) under the Stockholm Convention [13].
FCM legislation
In Europe, the ammonium salt of PFOA (APFO, CAS 3825-26-1) is authorized for the use in plastic FCMs intended for repeated use, which have to be sintered at high temperatures (Plastics Regulation EC 10/2011), but PFOS is not authorized under this regulation. In total, eight monomers and nine additives containing organically bound fluorine were identified on Annex I of the Plastics Regulation. The monomers (e.g. tetrafluroethylene (CAS 116-14-3) and hexafluoropropylene (CAS 116-15-4)) are mainly used for the production of perfluorinated polymers such as PTFE and fluoroethylenpropylen. The use of 14 of these substances is either restricted by a specific migration limit and/or by further specifications. In the EU, no harmonized legislations exist for most non-plastic FCMs, including paper and board. As a consequence, PFAS used in or on the surface of these materials are often not regulated and many of the substances have never been risk assessed by a public authority. Nonetheless, they share the persistence of PFOA and PFOS or may be degraded to substances with a similar persistence.
In the U.S., PFOA and PFOS are not listed in the U.S. FDA database of indirect food additives. However, this database lists 50 and 15 substances containing the search terms “fluor” and “perfluor”, respectively. They are regulated to be used in e.g. perfluorocarbon resins, paper and paperboard, components of resinous and polymeric coatings, and processing aids for polyolefins. In 2016, three long-chain PFAS that were previously regulated as indirect food additives were banned by the U.S. Food and Drug Administration (U.S. FDA) [17].
4. Exposure
PFAS are generally quantified in >95% of human samples all over the world, but they are also measured in almost all animal species and environmental samples [3, 18]. PFOA, PFOS and perfluorohexasulfonic acid (PFHxS) are the most abundant PFAS in humans [19]. Food, drinking water, indoor air and dust are major exposure sources of PFAS [18, 20] and FCMs contribute to the contamination of food [9, 21-23].
For the general population, exposure estimates for single PFAS are in the range of ng per kg body weight per day [3, 18, 24]. In contrast, PFAS manufacturing workers exceeded such exposure values by several orders of magnitude and elevated exposure levels were also reported for populations living in communities with PFAS-contaminated drinking water [25].
5. Health effects
In recent years, the health effects of PFOA and PFOS have thoroughly been investigated [26-31]. These chemicals are not only highly persistent and slowly eliminated from the human body, but they both cause various adverse health effects. Furthermore, their chemical derivatives may be degraded to PFOA and PFOS leading to even higher exposure levels.
Many fluorinated alternatives exist for PFOA and PFOS and are increasingly applied after efforts were undertaken to phase-out PFOA and PFOS. However, the toxicity of the substances used as substitutes is often not known [32].
Animal experiments showed that PFOA is hepatotoxic: It increases the liver weight, levels of cholesterol and liver enzymes, peroxisome proliferation and the histopathology [26, 27]. Further, it induces liver tumors, Leydig cell tumors, and pancreatic acinar cell hyperplasia. PFOA may reduce fecundity and fetal growth, increase neonatal mortality, affect the mammary gland development, and interfere with the immune system [29]. In 2011 and 2012, a panel of epidemiologists who were assigned after a court decision of a class-action lawsuit against DuPont (“C8-Panel”) concluded that PFOA is probably linked to kidney cancer, testicular cancer, ulcerative colitis, thyroid disease, hypercholesterolemia, and pregnancy-induced hypertension [33, 34]. A systematic review on the toxicity of PFOA concluded that PFOA is known to be toxic to human reproduction and development [35].
Exposure to PFOS led to decreased body weight and increased liver weight in animals [26, 28, 36]. It further affected the uric acid levels, lipid metabolism, the immune system, and the homeostasis of thyroid hormones [26, 29, 36]. There is evidence of carcinogenicity inducing tumors of e.g. the liver, thyroid, and mammary glands [26, 28]. Epidemiologic studies indicate associations between exposure to PFOS and high cholesterol levels, birth weight, liver function, and uric acid levels [37].
6. References
- Shin HM, Vieira VM, Ryan PB, et al. 2011. Retrospective exposure estimation and predicted versus observed serum perfluorooctanoic acid concentrations for participants in the C8 Health Project. Environ Health Perspect. 119:1760-5.
- Krafft MP, and Riess JG. 2015. Selected physicochemical aspects of poly- and perfluoroalkylated substances relevant to performance, environment and sustainability – Part one. Chemosphere. 129:4-19.
- Krafft MP, and Riess JG. 2015. Per- and polyfluorinated substances (PFAS): Environmental challenges. Curr Opin Colloid In. 20:192-212.
- Posner S, Roos S, Brunn Poulsen P, et al. 2013. Per- and polyfluorinated substances in the Nordic Countries – Use, occurence and toxicology. Nordic Council of Ministers. TemaNord 2013:542.
- Wang Z, Cousins IT, Scheringer M, et al. 2013. Fluorinated alternatives to long-chain perfluoroalkyl carboxylic acids (PFCAs), perfluoroalkane sulfonic acids (PFSAs) and their potential precursors. Environ Int. 60:242-8.
- Danish EPA. 2015. Short-chain polyfluoroalkyl substances (PFAS). Environmental project No. 1707.
- Lindström AB, Strynar MJ, and Libelo EL. 2011. Polyfluorinated compounds: past, present, and future. Environ Sci Technol. 45:7954-61.
- Siegemund G, Schwertfeger W, Feiring A, et al. 2012. Fluorine compounds, organic. In: Ullmann’s Encyclopedia of Industrial Chemistry. John Wiley and Sons, Weinheim. pp 493-94.
- Trier X, Granby K, and Christensen JH. 2011. Polyfluorinated surfactants (PFS) in paper and board coatings for food packaging. Environ Sci Pollut Res Int. 18:1108-20.
- Trier X, Nielsen NJ, and Christensen JH. 2011. Structural isomers of polyfluorinated di- and tri-alkylated phosphate ester surfactants present in industrial blends and in microwave popcorn bags. Environ Sci Pollut Res Int. 18:1422-32.
- Swedish Chemicals Agency. 2015. Occurrence and use of highly fluorinated substances and alternatives. Report 7/15.
- U.S. EPA. 2015. Per- and polyfluoroalkyl Substances (PFAS) under TSCA. [https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/and-polyfluoroalkyl-substances-pfass-under-tsca]
- Stockholm Convention on Persistent Organic Pollutants. 2016. Listing of POPs in the Stockholm Convention. [http://chm.pops.int/TheConvention/ThePOPs/ListingofPOPs/tabid/2509/Default.aspx]
- Zushi Y, Hogarh JN, and Masunaga S. 2012. Progress and perspective of perfluorinated compound risk assessment and management in various countries and institutes. Clean Technol Envir. 14:9-20.
- Benskin JP, Ikonomou MG, Gobas FA, et al. 2012. Observation of a novel PFOS-precursor, the perfluorooctane sulfonamido ethanol-based phosphate (SAmPAP) diester, in marine sediments. Environ Sci Technol. 46:6505-14.
- U.S. EPA. 2014. Emerging contaminants – perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA). Solid Waste and Emergency Response. EPA 505-F14-001.
- FDA. 2016. Indirect food additives: Paper and paperboard components. [https://www.federalregister.gov/articles/2016/01/04/2015-33026/indirect-food-additives-paper-and-paperboard-components]
- Fromme H, Tittlemier SA, Volkel W, et al. 2009. Perfluorinated compounds – exposure assessment for the general population in Western countries. Int J Hyg Environ Health. 212:239-70.
- Schröter-Kermani C, Müller J, Jürling H, et al. 2013. Retrospective monitoring of perfluorocarboxylates and perfluorosulfonates in human plasma archived by the German Environmental Specimen Bank. Int J Hyg Environ Health. 216:633-40.
- Skutlarek D, Exner M, and Farber H. 2006. Perfluorinated surfactants in surface and drinking waters. Environ Sci Pollut Res Int. 13:299-307.
- D’Eon JC, Crozier PW, Furdui VI, et al. 2009. Observation of a commercial fluorinated material, the polyfluoroalkyl phosphoric acid diesters, in human sera, wastewater treatment plant sludge, and paper fibers. Environ Sci Technol. 43:4589-94.
- Begley TH, White K, Honigfort P, et al. 2005. Perfluorochemicals: Potential sources of and migration from food packaging. Food Addit Contam. 22:1023-31.
- Rosati JA, Krebs KA, and Liu X. 2007. Emissions from cooking microwave popcorn. Crit Rev Food Sci Nutr. 47:701-9.
- EFSA. 2012. Perfluoroalkylated substances in food: occurrence and dietary exposure. EFSA Journal. 10:2743.
- Olsen GW. 2015. PFAS biomonitoring in higher exposed populations. In: Toxicological Effects of Perfluoroalkyl and Polyfluoroalkyl Substances. C.J. DeWitt, ed., Springer International Publishing, Cham. pp 77-125.
- EFSA. 2008. Opinion of the scientific panel on contaminants in the food chain on perfluorooctane sulfonate (PFOS), perfluorooctanoic acid (PFOA) and their salts. EFSA Journal. 653:1-131.
- U.S. EPA. 2014. Health effects document for perfluorooctanoic acid (PFOA) – Draft. 822R14001.
- U.S. EPA. 2014. Health effects document for perfluorooctane sulfonate (PFOS) – Draft. 822R14002.
- DeWitt JC, Peden-Adams MM, Keller JM, et al. 2012. Immunotoxicity of perfluorinated compounds: Recent developments. Toxicol Pathol. 40:300-11.
- White SS, Fenton SE, and Hines EP. 2011. Endocrine disrupting properties of perfluorooctanoic acid. J Steroid Biochem Mol Biol. 127:16-26.
- Olsen GW, Butenhoff JL, and Zobel LR. 2009. Perfluoroalkyl chemicals and human fetal development: An epidemiologic review with clinical and toxicological perspectives. Reprod Toxicol. 27:212-30.
- Wang ZY, Cousins IT, Scheringer M, et al. 2015. Hazard assessment of fluorinated alternatives to long-chain perfluoroalkyl acids (PFAAs) and their precursors: Status quo, ongoing challenges and possible solutions. Environ Int. 75:172-9.
- Fletcher T, Savitz D, and Steenland K. 2013. C8 Science Panel. [http://www.c8sciencepanel.org/index.html]
- Barry V, Winquist A, and Steenland K. 2013. Perfluorooctanoic acid (PFOA) exposures and incident cancers among adults living near a chemical plant. Environ Health Perspect. 121:1313-8.
- Lam J, Koustas E, Sutton P, et al. 2014. The Navigation Guide – evidence-based medicine meets environmental health: integration of animal and human evidence for PFOA effects on fetal growth. Environ Health Perspect. 122:1040-51.
- Lau C, Anitole K, Hodes C, et al. 2007. Perfluoroalkyl acids: A review of monitoring and toxicological findings. Toxicol Sci. 99:366-94.
- Gleason JA, Post GB, and Fagliano JA. 2015. Associations of perfluorinated chemical serum concentrations and biomarkers of liver function and uric acid in the US population (NHANES), 2007-2010. Environ Res. 136:8-14.
